Working Group ‒ a Portrait:
Institute for Stem Cell Research and Regenerative Medicine (ISRM)
at Heinrich Heine University Düsseldorf
Director of the ISRM is the biochemist and molecular biologist Prof. Dr. James Adjaye. He worked for many years in the United Kingdom before specialising in molecular embryology and stem cell research at the Max Planck Institute for Molecular Genetics in Berlin. Since May 2012, Prof. Adjaye has been Director of the Institute for Stem Cell Research and Regenerative Medicine, University Hospital of Düsseldorf (UKD), Germany.
His research team uses a systems biology approach to investigate both the normal development of the hepatic and renal systems and ageing processes and disease mechanisms, ranging from Alzheimer’s disease to non-alcoholic fatty liver disease. He was also Group Leader of the Molecular Embryology and Aging Group, Department of Vertebrate Genomics at the Max Planck Institute for Molecular Genetics in Berlin for many years, as well as the coordinator the transnational research consortium project ERASysBio Plus. 16 projects from 13 countries were linked up with the goal of developing mathematic models and prediction programs (1). One of these programs investigated the aetiology of non-alcoholic fatty liver disease. ERASysBio Plus was funded by the German Federal Ministry of Education and Research.
Fatty liver diseases very widespread in industrialised societies
The proportion of obese people in the world’s population is on the rise*: A recent study published in the prestigious journal The Lancet predicts that global obesity will reach 18 per cent of men and 21 per cent of women by the year 2025 (2). Along with the rise in obesity, the share of those who may suffer from a fatty liver disease will also rise, whereby there are also other causes (3). According to current estimates, a sedentary lifestyle and an excessively fat-rich diet in Western industrialised societies will lead to fatty liver conditions in approximately 30 per cent of the population (4).
Other diseases can also lead to a fatty liver (secondary steatosis, 5). This can be caused by poorly controlled diabetes, a lipid metabolism disorder resulting from abnormal lipoprotein synthesis1, a hepatitis C virus infection or malnutrition (6, 5). Genetic factors that can play a part can for instance be genetic alterations for the proteins PNPLA3 and TM6SF2 (7). A single amino acid exchange in the protein PNPLA3 leads to a predisposition for a serious fatty liver disease. Normally the protein regulates the lipid metabolism in liver and fat tissues (8). There is a comparable situation with regard to the protein TM6SF2, which is present in the liver and the intestine and also plays a part in lipid metabolism (9).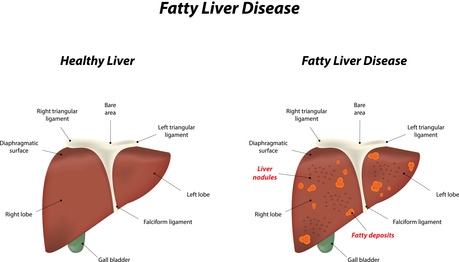
A fatty liver displays distinct lipid deposits, mostly comprising triglycerides.
Photo: Joshya, Fotolia.com
Signs of a non-alcoholic fatty liver are accumulations of lipids (especially triglycerides) in the liver cells (hepatocytes). A mild grade fatty liver affects less than 33 per cent of the liver’s storage cells, whereas a severe grade fatty liver affects more than 66 per cent of the storage cells. As long as there are no fibrosis2 and no inflammation, a fatty liver disease is reversible (5).
Fatty liver disease is considered to be responsible for 10-20 per cent of all liver cirrhoses3 and all forms of hepatocellular carcinoma (HCC) in industrialised societies (5).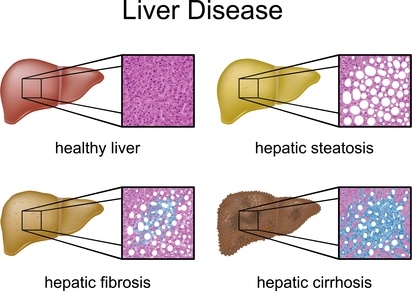
Different stages of fatty liver disease. Whilst no lipid deposits are visible in a healthy liver (first illustration), a liver affected by steatosis is already riddled with lipid accumulations (top right illustration). The steatosis is without external complications, however the risk of a myocardial infarction is elevated (4). This stage is still reversible, as opposed to hepatic fibrosis (third picture, bottom left). In the stage of hepatic fibrosis, inflammation processes lead to connective tissue scarring (marked blue). The final stage is hepatic cirrhosis (shrunken liver, bottom right), with the liver unable to fulfil its function, because the liver tissue that was previously parenchyma has been transformed into functionless connective tissue.
Photo: Peter Junaidy, Fotolia.com
Non-alcoholic fatty liver disease (NAFLD)
Non-alcoholic fatty liver disease is a when a liver has fat deposits that make up 5 to 10 per cent of the liver’s weight, and these are not due to increased alcohol consumption. The prevalence of NAFLD in the general population is approx. 20-30 per cent worldwide (5).
Non-alcoholic fatty liver disease includes many diseases, ranging from steatosis without inflammation processes or notable progression to non-alcoholic fatty liver disease with inflammation and hepatocyte damage (steatohepatitis) resulting in severe proliferation of connective tissue and finally fibrotic scarring. In certain cases hepatic fibrosis can culminate in hepatic cirrhosis, with a shrunken liver and the death of liver tissue. It is estimated that up to 0.3 per cent of the general population develops hepatic cirrhosis. Liver cancer can also ensue.
Non-alcoholic fatty liver disease can be the result of metabolic disorders, obesity, poorly controlled diabetes mellitus, chronic inflammatory intestinal diseases or side effects of pharmaceutical drugs (3,10).
Thus far there is still no classification of risks for fatty liver disease. This requires knowledge of the conditions that negatively affect the disease (11). The scientists therefore want to use their model to track down the mechanisms that cause the disease and affect its progress. To this end they intend to identify suitable biomarkers and after evaluation of genome-wide association studies (GWAS)4 develop new diagnostic tools for fatty liver disease (4).
Induced pluripotent stem cells: from somatic cells to disease in a dish
This multifactorial liver disease can be investigated using both animal models (10) and cell culture models in a Petri dish, so-called disease in a dish models. However, due to the differences in disease phenotypes between humans and animals, the results of research on animals cannot always be reliably applied to the human condition.
There are animal models that are used for investigating fatty liver disease, but they cannot truly map the processes, as the causes are so varied and can only be artificially induced in animals using one or two factors.
Various mouse models have been developed, but none of these completely reflects the histopathology of the liver or the pathophysiology of human non-alcoholic fatty liver disease (10). For example, researchers use a spontaneous mutation in the gene that codes leptin in mice; leptin is a hormone produced in white fatty tissue and the mutation causes the animals to develop extreme obesity, insulin resistance and elevated insulin levels, but not steatohepatitis, i.e. they have a fatty liver, but do not spontaneously develop additional inflammation the way humans do. Other models developed liver fibrosis when, in addition to the mutation, the animals were fed a diet deficient in the essential amino acid methionine and the vitamin-like substance choline (10). In humans the intake of choline is not essential, as long as the diet contains methionine and folic acid (12).
Important mechanisms for the development of fatty live disease can also be investigated in vitro instead of using animals. The stem cell researcher Prof. James Adjaye cultivated the first German induced pluripotent stem cells (iPS cells) at the Max Planck Institute for molecular Genetics in Berlin in 2008. This is the ethical solution for the use of human stem cells, as using human embryonic stem cells is considered morally questionable in Germany.
Producing induced pluripotent stem cells
The production of iPS cells requires only a small cell sample, for instance obtained non-invasively from skin, umbilical cords or urine. Cells can be also obtained invasively from bone marrow. The methods employed so far require the introduction of four specific genes (Oct4, Sox2, c-Myc und Klf4) into the cell in order to reprogram it (Takahashi & Yamanaka 2006, 13, 14). Viruses are used as vehicles for introducing the genes that penetrate and alter the cell’s genome. The genetic alteration can, however, lead to genetic anomalies and even to an elevated risk of cancer (15). Various research groups are however working intensively on possible solutions.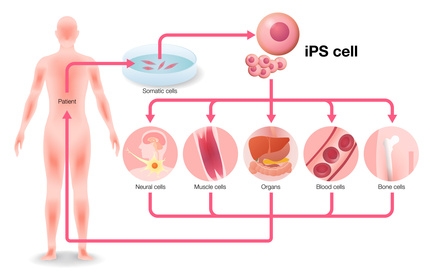
Human somatic cells, e.g. skin cells, mesenchymal stroma cells5 or cells from umbilical cord blood can be reprogrammed to embryonic cells and then differentiated into the desired cell type. Methods used so far introduced four specific genes (Oct4, Sox2, c-Myc und Klf4) into the cell, using viruses as vehicles for introducing the genes that penetrate and alter the cell’s genome. The genetic alteration can, however, lead to genetic anomalies. A more advanced method introduces biotechnologically created proteins into the cells in order to reprogram them. The scientists are now concentrating on the so-called chromatin regulators. This increases the cell yield and reduces the production duration for iPS cells from nine to four days. Once reprogrammed, the cells can be differentiated into the desired cell type, whether nerve, muscle, blood or bone cells. This allows the construction of cells and even organ-like structures that are not only human-specific but also carry a patient’s genetic information.
Photo: chombosan, Fotolia.com
So far a more advanced method has been not to alter the genes but instead to introduce certain biotechnologically created proteins into the cells (so-called protein-induced pluripotent stem cells, 16) or to use so-called small molecules (up to 800 g/mol), whose small size allows them to penetrate the cells. They are not proteins but can dock onto receptors and initiate or interrupt signal cascades (16, 17). Other researchers are convinced they have found the cells‘ own switch for reprogramming them to embryonic cells. They are concentrating on the so-called chromatin regulators – factors responsible for the cells’ epigenetic memory. Amongst the 615 factors an innovative research team tested, four factors seem definitely responsible for maintaining the cells‘ differentiation and preventing the formation of iPS cells. The four factors are SET domain-containing H3K9 methyltransferase (Setdb1), CHAF1A and CHAF1B – responsible for forming the chromatin assembly factor 1 complex – and ubiquitin conjugating enzyme 2I (18). When these factors were suppressed in combination, the scientists obtained an iPS cell yield from the culture 50 to 2000 times higher than with a single factor. This new discovery enabled the production time for iPS cells to be reduced from nine to four days (19).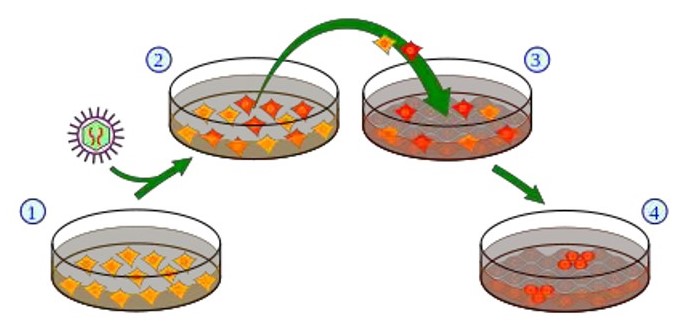
Classic method for producing induced pluripotent stem cells.
1. Cell culture using somatic cells, 2. Introduction of the pluripotency genes into the cells using a retrovirus vector. Cells that express the exogenous genes are depicted in red. 3. Harvest and cultivation of the cells using “feeder calls” (connective tissue cells, so-called fibroblasts [grey]). 4. A small subset of the cells (red) generates iPS cells.
Scheme: Y tambe, Wikipedia.
Human liver and heart cells generated using iPS cells are currently suitable for screening potential pharmaceutical drugs for toxic side effects. Since cardiotoxic pharmaceuticals must be eliminated from further development, such screening can rule out unsuitable substances from the start without having to test them on animals at all (reduction of animal experiments).
Their human-specificity also allows the harvested cells to be used for finding patient-specific therapeutic substances and investigating their mechanisms of action (20). iPS cells are now already being used in clinical studies as cell replacement therapies for age-related macular degeneration (21).
One potential use could be growing patients’ own organs as a response to a shortage of donor organs.
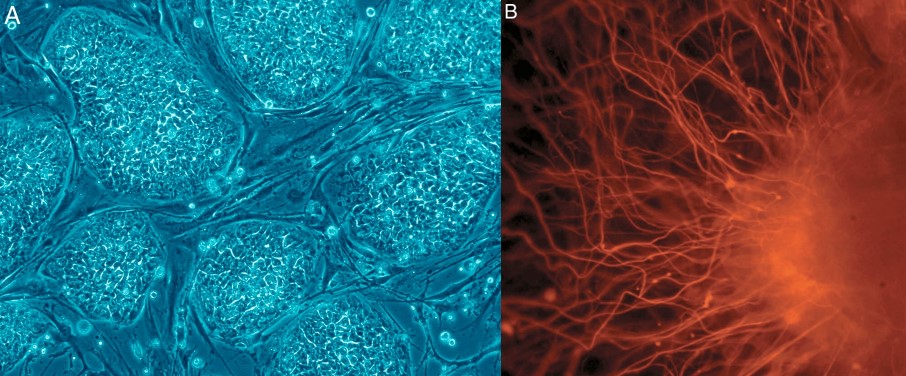
A: Human embryonic stem cells (hESCs) and B: nerve cells derived from them.
Photo: Nissim Benvenisty.
Success of cells’ pluripotency no longer needs verification by the teratoma test
Until recently, the researchers used mice to test whether the cells displayed the desired pluripotency characteristics. Pluripotency means that the cells can be developed to become all three germ layers that make up all organs: endoderm, mesoderm and ectoderm. The cells to be tested are injected under the skin of the mice. If a tumour – a so-called teratoma – develops, in which all three germ layers can be proven, the cells are regarded as pluripotent. Mostly immunodeficient mice are used under sterile conditions, with the mice being subjected to considerable suffering not only during but also before the experiments. (22). A thorough investigation can use 50-100 mice per test. There are no uniform criteria for conducting the Test (23) and the results can only be ensured by a highly specialised pathologist.
There are several cheaper and quicker animal-free alternatives to the teratoma test for verifying the pluripotency of human stem cells, although they are not all equally suitable. For instance, there is the proof of active pluripotency genes by studying DNA methylisation patterns, proof of marker proteins (suitable for single investigations) or proof of directed differentiation in cells of the three germ layers. Pluripotency of IPS cells from animals can be proven using a number of other test alternatives.
Prof. Adjaye’s working group prefers the PluriTest (23). This is a bioinformatic assay that uses the data from gene expression profiles for evaluating pluripotency based on human induced pluripotent stem cells and not animal pluripotent cells. A corresponding database contains some 450 gene expression data sets (SCM2 database). The data sets were generated with microarrays using numerous stem cell preparations from different laboratories and also contain gene expressions of differentiated cell types, and developing or differentiated human tissues. The data sets also include stem cell expression profiles from both human embryonic stem cells and induced pluripotent stem cell lines.
Example of the results of a DNA microarray. The brighter the dot, the more messenger was produced for a certain gene. This kind of gene chip is used for generating gene expression profiles that are the basis for PluriTest.
Photo: Mangapoco.
First, the program uses a mathematical regression model to assign pluripotency points, with which all samples and the already contained gene expression profiles are classified and can distinguished as pluripotent und non-pluripotent. After that a ranking of the correlation between the number of mutagens and already known pluripotent or non-pluripotent samples is determined. The second calculation consists of a non-negative matrix factorisation model (NMF model), a tool with a group of algorithms from multivariate statistics and linear algebra (24).
Systems biology – requisite computer assistance
As biological processes are extremely complicated and possess a multitude of mutually influencing components, many questions can no longer be answered using the methods of classic biology. Also, the plethora of data generated by countless measurements worldwide can only be evaluated and made available with the help of powerful computers.
Systems biology combines methods from biology, mathematics, computer sciences, physics and engineering and develops mathematical models as hypotheses that are then repeatedly tested and refined with new data. Scientific questions that arise can lead to new scientific experiments (25).
We interviewed the stem cell specialist Prof. Dr. Adjaye about his work and the current status of research on induced pluripotent stem cells.
InVitro+Jobs: For a long time, you have worked at the Max Planck Institute for Molecular Genetics in Berlin - one can say you are one of the first breeder of iPS cells in Germany, isn´t it right?
Prof. Adjaye: I was group leader from 2001 till 2014 at the Max Planck Institute for Molecular Genetics in Berlin. Yes, my lab was amongst the first groups in Germany to publish derivation of iPS cells from human somatic cells.
InVitro+Jobs: Are there all significant obstacles to reprogramming of iPS cells have now been overcome?
Prof. Adjaye: At the moment reprogramming protocols have become routine, not using retro-6 or lenti-7 viruses. Some labs still use Sendai8 viruses but we only use non-integrative plasmids9 and this works well.
InVitro+Jobs: What difficulties exist today?
Prof. Adjaye: mRNA-based reprogramming protocols are still a challenge.
InVitro+Jobs: Is the exact mechanism of reprogramming of iPS known indeed?
Prof. Adjaye: Yes, a lot is known but the focus and trend now is to understand why chromosomal aberrations10 occur in iPS cells, especially when derived from cells originating from aged individuals. This is a very important issue especially if iPS cells are to be used routinely in the future for cell-based therapy.
InVitro+Jobs: Many researchers are working with animal models: What makes you sure that iPS cell models are the "major road"? What kind of important role play iPS cells in the development of replacement method to animal experiments?
Prof. Adjaye: The obvious advantage of iPS technology is that we can study human diseases and this is very important because there are differences in disease phenotypes in rodents and human. We cannot always reliably extrapolate findings in rodents to the human condition.
InVitro+Jobs: Why do you investigate e.g. the mechanisms of the non-alcoholic fatty liver in vitro? What are the advantages of disease models on a dish compare to a fatty liver animal model?
Prof. Adjaye: NAFLD is a silent epidemic of the 21st century, 1 in 3 people in western societies is obese and it is estimated that 30% of the population suffer from NAFLD. Of course till now most studies have been conducted in rodents and those in human are mainly epidemiology or screening in serum.
Because NAFLD is also a life style disease (fatty diets and lack of adequate exercise) and there are differences in susceptibility between individuals (Some people have fatty diets but are not obsese, so there must be a genetic component involved).
For all these reasons and iPSC-based study is more appropriate because we have skin cells (iPS cells), serum and also clinical data from our patients so we can have a better understanding of disease mechanisms. Using iPS cells we can also induce the disease by differentiating the cells into hepatocytes and then induce steatosis by adding oleic acid to the cell culture and analyse downstream11 molecular events such as gene regulation and altered disease- associated pathways.
InVitro+Jobs: Do you think that in vitro disease models will be suitable to replace animal testing in distant future, maybe in combination with the human-on-a-chip models13?
Prof. Adjaye: Total replacement, I do not think so. In vitro disease models would help reduce and refine animal testing and of course human-on-a –chip will also be useful in this regard.
InVitro+Jobs: How do you assess the problem of the use of fetal calf serum in stem cell research?
Prof. Adjaye: We do not use this due to associated problems of unknown/not defined factors and batch to batch variations14.
InVitro+Jobs: How do you evaluate on of the alternatives to the teratoma assay with mice, the PluriTest? Is it applicable to replace the teratoma test well with mice?
Prof. Adjaye: Absolutely, we have stopped performing the teratoma assay, rather we carry out the EB assay in vitro and then the pluritest.
InVitro+Jobs: Do you know other replacement methods to the teratoma test?
Prof. Adjaye: Only pluritest is realiable.
InVitro+Jobs: Can you describe something about the embryoid body15-based assays to identify the pluripotency? Is it now possible to induce pluripotency via small molecules?
Prof. Adjaye: We are still trying. A few years back we achieved this using 1st trimester amniotic fluid cells, these are very young cells and difficult to obtain. So far no one has successfully and reproducibly induced pluripotency in human somatic cells using only small molecules. I do not think this is impossible, we are currently working actively on this.
InVitro+Jobs: How many staff members are working in your lab?
Prof. Adjaye: 15 and more are expected.
InVitro+Jobs: Can you offer thesis assignments or jobs in the field of stem cell research?
Prof. Adjaye: Yes, to bright students.
InVitro+Jobs: Thank you for the interview.
* determined using the Body Mass Index (BMI)
Glossary:
1 Lipoprotein synthesis: Water-insoluble lipids bind to protein, creating lipoproteins that are transported in the blood for the purpose of lipid metabolism.
2 Fibrosis: Inflammation processes lead to scarring by connective tissue.
3 Liver cirrhosis: Shrunken liver. Storage tissue has been transformed into functionless connective tissue.
4 Genome-wide association studies (GWAS): The DNA of a large number of patient samples is compared with that of healthy probands at the relevant positions.
5 Mesenchymal stroma cells: Supporting and connective tissue that can be reprogrammed to generate bone-building cells, cartilage cells and fat cells.
6 Retroviruses: RNA viruses with reverse transcriptase. Once the RNA penetrates the host cell, this enzyme rewrites it to double-stranded DNA. Another viral enzyme (integrase) forms a loop with OH groups at the ends.". These attacks the host cell at the target chromosomes, integrating the viral DNA into the host chromosome. Remaining damage to the host DNA is removed using the host cells own repair enzyme.
7 Lentiviruses: Single-strand RNA viruses. Can also infect cells that do not divide, such as nerve cells.
8 Sendai viruses: The Sendai virus is the murine parainfluenza virus, type 1. It is a single-strand RNA virus that requires no DNA phase for its replication, which means that . This means that using them for generating iPS cells avoids breaking the host cells’ DNA strands (26).
9 Non-integrating plasmids: Plasmids that cannot be built into the genomes of cells.
10 Chromosome aberrations: Structural alteration of chromosomes or chromatids, for instance by increasing the number of chromosomes (numerical aberration) or altering their shape by breaking or falsely connecting parts of chromosomes (structural aberration). Chromosome parts can be deleted, inverted or fused to rings.
11 Downstream: Biochemical expression. Cell reactions to a stimulus run through a signal cascade, in which a receptor detects a stimulus outside the cell and the stimulus is transmitted via various cellular processes, such as the altered configuration of molecules or the binding small molecules such as phosphate groups, until it reaches the cell nucleus, where in the DNA one or genes for producing proteins are read. Thus the cell reacts to the stimulus. The beginning of the cascade is known as “upstream”, the course further down as “downstream”.
12 Signal transduction pathway: see „Downstream“
13 Human-on-a-chip models: A number of scientists intend to combine at least 10 miniaturised human organs on a microchip by the year 2018 and be able to use this miniature organism to replace many animal experiments.
14 Batch-to-batch variation: The use of inexactly characterised media can lead to a variation in the results delivered by different batches, which should be prevented (27).
15 EB Assay: Embryoid Body Assay. Unlike the teratoma test using mice, the Embryoid Body Assay is an in-vitro assay that tests the ability of cells to aggregate when the stem cells are cultured without contact to a surface. In spherical form they emulate embryonic development in a limited fashion by generating single cells in all tissue types (mesoderm cells, haematopoietic cells, endothelial cells,, muscle cells and neural cell lines). in 1999 Leahy et al. suggested using early expression of marker genes for identification (28) (for a description of the teratoma test see the main text).
Literature and Sources:
(1) https://www.erasysbio.net/index.php?index=263
(2) NCD Risk Factor Collaboration (NCD-RisC) (2016): Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1.698 population-based measurement studies with 19,2 million participants. Lancet 387: 1377–96.
http://www.thelancet.com/journals/lancet/article/PIIS0140- 6736%2816%2930054-X/fulltext
(3) http://www.aerzteblatt.de/archiv/160842
(4) Wruck, W., Kawala, M.-A., Graffmann, N. & Adjaye, J. (2016): Strategies for identifying predictive biomarkers of non-alcoholic fatty liver disease. http://www.drugtargetreview.com/10985/content- type/articles/strategies-identifying-predictive-biomarkers-non-alcoholic-fatty-liver-disease/
(5) Roeb E, Steffen HM, Bantel H, et al. (2015): S2k Leitlinie Nicht-alkoholische Fettlebererkrankungen. Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. (AWMF)-Register Nr. 021-025. http://www.awmf.org/uploads/tx_szleitlinien/021- 025l_S25_NASH_Nicht_alkoholische_Fettlebererkrankung_2015-01.pdf
(6) Buddecke, E. & Fischer, M. (1992): Pathophysiologie, Pathobiochemie, Klinische Chemie. Walter de Gruyer Verlag, Berlin, New York.
(7) Dongiovanni, P., Donati, B., Fares, R., et al. (2013): PNPLA3 I148M polymorphism and progressive liver disease. World J Gastroenterol 19 (41): 6969-6978. http://www.wjgnet.com/esps/
(8) Berger, C., Luda, C., Berg, T. et al. (2011): PNPLA3 (rs738409) 148M/M Homozygosität: ein genetischer Risikofaktor für hepatozelluläre Karzinome bei äthyltoxischer, jedoch nicht bei HCV- assoziierter Leberzirrhose. Z Gastroenterol 49 - P308 DOI: 10.1055/s-0031-1285579. https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0031-1285579
(9) Mahdessiana, H., Taxiarchisa, A., Popova, S., et al. (2014): TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. PNAS 111/24: 8913– 8918. http://www.pnas.org/cgi/doi/10.1073/pnas.1323785111
(10) Takahashi, Y., Soejima, Y. & Fukusato, T. (2012): Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 18 (19): 2300-2308.
(11) http://flexikon.doccheck.com/de/Risikostratifikation
(12) Zeisel, S. H., Da Costa, K. A., Franklin, P. D., et al. (1991): Choline, an essential nutrient for humans. FASEB Journal 5/7: 2093–2098. http://www.ncbi.nlm.nih.gov/pubmed/2010061
(13) http://www.eurostemcell.org/de/factsheet/reprogrammierung-wie-jede-zelle-des-k%C3%B6rpers- zu-einer-pluripotenten-stammzelle-gemacht-wird
(14) Kazutoshi Takahashi & Shinya Yamanaka (2006): Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 126: 663–676.
(15) http://www.stammzellen.nrw.de/ueber-stammzellen.html
(16) Cheng, X., Dimou, E., Alborzinia, H., et al. (2015): Identification of 2-[4-[(4-Methoxyphenyl)methoxy]- phenyl]acetonitrile and Derivatives as Potent Oct3/4 Inducers. J. Med. Chem. 2015, 58, 4976-4983. DOI: 10.1021/acs.jmedchem.5b00144
(17) Lin, T, et al. (2009): A chemical platform for improved induction of human iPSCs. Nat Methods 6(11): 805-808.
(18) Cheloufi, S., Elling, U., Hopfgartner, B., et al. (2015): The histone chaperone CAF-1 safeguards somatic cell identity. Nature 528: 218-224. doi:10.1038/nature15749. http://www.nature.com/nature/journal/v528/n7581/full/nature15749.html
(19) http://www.ipt.fraunhofer.de/de/presse/Pressemitteilungen/20160307autostem.html
(20) Mlody, B. & Adjaye, J. (2015): Generation of iPSC lines from a Nijmegen Breakage Syndrome patient. Stem Cell Research 15: 629–632.
(21) Alessandro Prigione • Marı´a Victoria Ruiz-Pe´rez et al. (2015): Metabolic restructuring and cell fate conversion. Cell. Mol. Life Sci. 72: 1759–1777. DOI 10.1007/s00018-015-1834-1
(22) Buta, C., David, R., Dressel, R., et al. (2013): Reconsidering pluripotency tests: Do we still need teratoma assays? Stem Cell Research 31/11: 552-562. http://www.sciencedirect.com/science/article/pii/S1873506113000275
(23) Müller, F.-J., Schuldt, B. M., Williams, R., et al. (2011): A bioinformatic assay for pluripotency in human cells. Nature Methods 8/4: 315-317. http://www.nature.com/nmeth/journal/v8/n4/abs/nmeth.1580.html
(24) Dhillon, I., Sra, S. (2005): Generalized Nonnegative Matrix Approximations with Bregman Divergences. In: Neural Information Processing Systems (NIPS): 283-290.
http://bigdata.ices.utexas.edu/publication/generalized-nonnegative-matrix-approximations-with- bregman-divergences/
(25) https://www.systembiologie.de/de/foerderung/sytembiologiefoerderung
(26) Kishino, Y., Seki, T., Yuasa, S., et al. (2015): Generation of Induced Pluripotent Stem Cells from Human Peripheral T Cells Using Sendai Virus in Feeder-free Conditions. J. Vis. Exp. (105), e53225, doi:10.3791/53225. http://www.jove.com/video/53225/generation-induced-pluripotent-stem-cells- from-human-peripheral-t
(27) Gerth, U. & Schöler, H. (2014): Lost in Translation? Laborjournal online.
http://www.laborjournal.de/j20/j_11.lasso
(28) Leahy, A., Xiong, J. W., Kuhnert, F. & Stuhlmann, H. (1999): Use of developmental marker genes to define temporal and spatial patterns of differentiation during embryoid body formation. J Exp Zool. 284/1: 67-81.
Recommended publications of interest:
Hossini, A. M., Megges, M., Prigione, A., et al. (2015): Induced pluripotent stem cell-derived neuronal cells from a sporadic Alzheimer’s disease donor as a model for investigating AD-associated gene regulatory networks. BMC Genomics 16/84. DOI 10.1186/s12864-015-1262-5
Jozefczuk, J., Prigione, A., Chavez, L. & Adjaye, J. (2011): Comparative analysis of human embryonic stem cell and induced pluripotent stem cell-derived hepatocyte-like cells reveals current drawbacks and possible strategies for improved differentiation. Stem Cells Dev (7): 1259-75. doi: 10.1089/scd.2010.0361.
Matz, P. & Adjaye, J. (2015): Generation of iPSC line epiHUVEC from human umbilical vein endothelial cells. Stem Cell Research 15: 581–583.
Megges, M., Geissler, S., Duda, G. N. & Adjaye, J. (2015): Generation of an iPS cell line from bone marrow derived mesenchymal stromal cells from an elderly patient. Stem Cell Research 15: 565–568.
Mlody, B. & Adjaye, J. (2015): Generation of iPSC lines from a Nijmegen Breakage Syndrome patient. Stem Cell Research 15: 629–632.
Mockus, L., Peterson, J. J., Lainez, J. M., et al. (2015): Batch-to-Batch Variation: A Key Component for Modeling Chemical Manufacturing Processes. Org. Process Res. Dev. 19: 908−914.
Moschidou, D., Mukherjee, S., Blundell, M. P., Drews, K., Jones, G. N., Abdulrazzak, H., Nowakowska, B., Phoolchund, A., Lay, K., Ramasamy, T. S., Cananzi, M., Nettersheim, D., Sullivan, M., Frost, J., Moore, G., Vermeesch, J. R., Fisk, N. M., Thrasher, A. J., Atala, A., Adjaye, J., Schorle, H., De Coppi, P. & Guillot, P. V. (2012): Valproic acid confers functional pluripotency to human amniotic fluid stem cells in a transgene-free approach. Mol Ther (10): 1953-67. doi: 10.1038/mt.2012.117.
Nishikawa, S.-I., Goldstein, R. A. & Nierras, C. R. (2008): The promise of human induced pluripotent stem cells for research and therapy. Nature Reviews Molecular Cell Biology 9: 725-729.
Prigione, A. & Adjaye, J. (2014): A mitochondrial strategy for safeguarding the reprogrammed genome. Cell Regeneration: 3/5. http://www.cellregenerationjournal.com/content/3/1/5
Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. (2010): The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. (4):721-33. doi: 10.1002/stem.404.
Prigione, A., Rohwer, N., Hoffman, S., Mlody, B., Drews, K., Bukowiecki, R., Blümlein, K., Wanker, E. E., Ralser, M., Cramer, T. & Adjaye, J. (2014): HIF1α modulates reprogramming through early glycolytic shift and up-regulation of PDK1-3 and PKM2. Stem Cells. 32(2):364-76.
Prigione, A., Ruiz-Pérez, M. V., Bukowiecki, R. & Adjaye, J. (2015): Metabolic restructuring and cell fate conversion. Cell. Mol. Life Sci. 72: 1759–1777.
Schröter, F., Sleegers, K., Cuyvers, E., et al. (2016): Lymphoblast-derived integration-free iPS cell line from a 65-year-old Alzheimer's disease patient expressing the TREM2 p.R47H variant. Stem Cell Research 16: 113–115.
Schröter, F., Sleegers, K., Bohndorf, M. (2016): Lymphoblast-derived integration-free iPS cell line from a 69-year-old male. Stem Cell Research 16: 29–31.
Stachelscheid, H., Wulf-Goldenberg, A., Eckert, K., Jensen, J., Edsbagge, J., Björquist, P., Rivero, M., Strehl, R., Jozefczuk, J., Prigione, A., Adjaye, J., Urbaniak, T., Bussmann, P., Zeilinger, K. & Gerlach, J. C. (2013): Teratoma formation of human embryonic stem cells in three-dimensional perfusion culture bioreactors. J Tissue Eng Regen Med (9): 729-41. doi: 10.1002/term.1467.
Wruck, W., Kashofer, K., Rehman, S., et al. (2015): Multi-omic profiles of human nonalcoholic fatty liver disease tissue highlight heterogenic phenotypes. DOI: 10.1038/sdata.2015.68.
http://www.nature.com/sdata/
