TissUse is a team of scientists. Scientific founder, CEO and head of the “Multi-Organ-Chip” programme is the physician and biologist Dr. Uwe Marx; In addition to TissUse, Marx has co-founded to other successful enterprises: ProBioGen AG and VITA34. The team also includes Dr. Reyk Horland, Head of Business Development and specialised in biotechnology. The biochemist Dr. Silke Hoffmann is the third member of the team and responsible for marketing intellectual property and patent law issues. She is an expert in the area of commercial property rights. In total, the team comprises 12 staff members/colleagues (as of July 2015 according to TissUse).

Ill. 1: TissUse founder Uwe Marx (right) with his co-workers.
Photo: Lutz Maternowski.
Of course, these three could not shoulder the comprehensive developments on their own. There are diverse co-operations, for instance with the Technische Universität Berlin, led by Prof. Dr. Roland Lauster and his team at the Chair of Medical Biotechnology; the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB in Stuttgart; the Fraunhofer IWS in Dresden or the University of Würzburg.
What is special is that each scientist or research institute is invited to collaborate on the development of the multi-organ chip.
TissUse1 was founded in 2010 as a spin-off of the Technische Universität Berlin. Here the idea of a human organism with the dimensions of a microscope slide was further developed and commercialised.
Ill. 2: TissUse is sited in a historic AEG factory in Berlin-Wedding, seen here from the Gustav-Meyer-Allee.
Photo: Frisia Orientalis.
Modelling the Human Organism – “Human-on a-Chip“
Since 2013 the scientists have been able to connect two organs on a chip with each other and to offer the “2-organ chip” for commercial use. The two-organ platform is already used by more than 20 scientific facilities and industrial research groups such as Beiersdorf. The commercialisation is supported by 4.4 million euros in funding from the German Federal Ministry of Education and Research within the framework of the initiative “GoBio”.
The two-organ platform comprises either skin and liver, skin and immune system or another organoid combined with the liver, for instance nerve tissue. The liver organoid is often used in the chips as a primary metabolic organ, as the liver can detoxify one test substance in the human organism but also cause another substance to become toxic in the first place. The connection between the organs takes place with the help of micro-pumps, channels and membranes. The microorganisms already deliver results that allow predictions of the natural reactions of human organs, such as side-effects to drugs, cosmetics, chemicals or other products, and could thus already reduce animal tests.
Last year, Dr. Uwe Marx received the Animal Welfare Research Prize awarded by the German Federal Ministry of Food and Agriculture for developing the system2.
In a real organism, a substance passes through the body. Once it has been ingested orally and taken in via either the gastric or intestinal mucosa, it is transported via the hepatic portal vein to the liver, where it is metabolised. Part of it is destined for excretion, i.e. does not even reach the target organ. The “rest” is transported from the liver back into the circulatory system and on to the target organ. A cell culture, which often comprises only one cell type, would of course be unsuitable for this important analysis.
Systemic Toxicology
Systemic Toxicology investigates the distribution of a substance in the body. The substance can be ingested orally, absorbed dermally or for instance administered intravenously. Often a target organ can be damaged or influenced in the process.
The organism’s exposure is classified according to duration.
Acute toxicology refers to a single exposure, whereas subacute limits the exposure to between a fortnight and a month.
One speaks of chronic toxicology when the organism is permanently exposed to the substance, whereas subchronic means an exposure of for instance three months to a year.
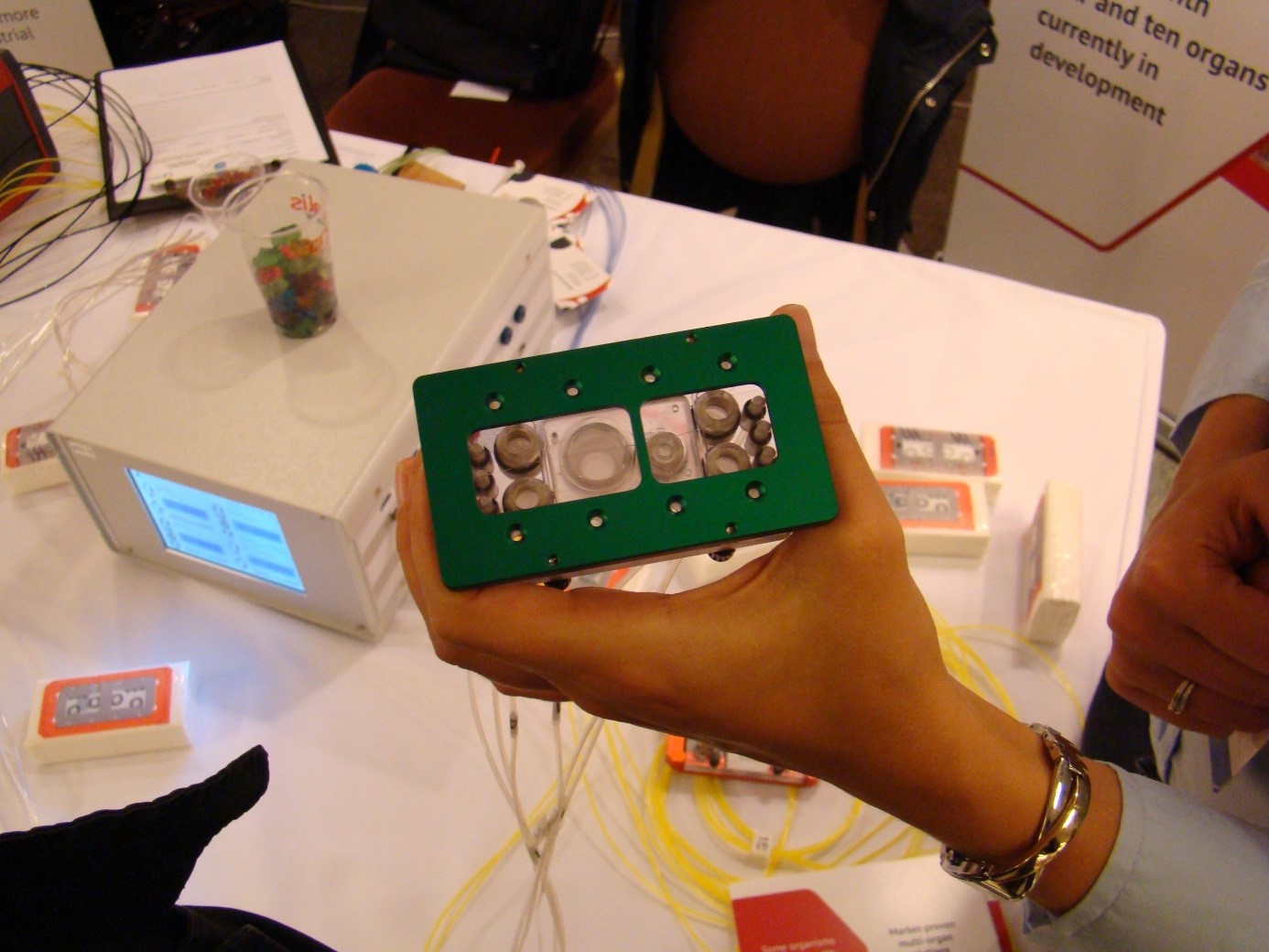
Ill. 3: The 4-organ chip from TissUse.
Photo: Christiane Hohensee
Such cell cultures nonetheless have an important function, for instance when screening substances for their potential suitability. Cell cultures are also significant for mechanistic investigation of the reaction or the biochemistry of neighbouring cells.
Since the end of 2014, the scientists at TissUse have been able to connect four organoids on a chip: Intestine, liver, kidney and skin are connected via minute micro-channels and are supplied by a blood-like system. This ADME chip (ADME= Absorption, Distribution, Metabolism, Excretion) already allows toxicological tests comparable to those conducted on animals to investigate the harmful effects of a substance when applied repeatedly. These platforms thus already make a reduction of animal tests possible.
The goal is to represent ten or more human organs on the chip platform from mid-2018.
Intestine, liver, pancreas, kidneys, skin, ovaries/testes, brain, fat tissue, bone marrow, blood system and other organs such as the spleen, with the organs arranged according to their physiological properties3.
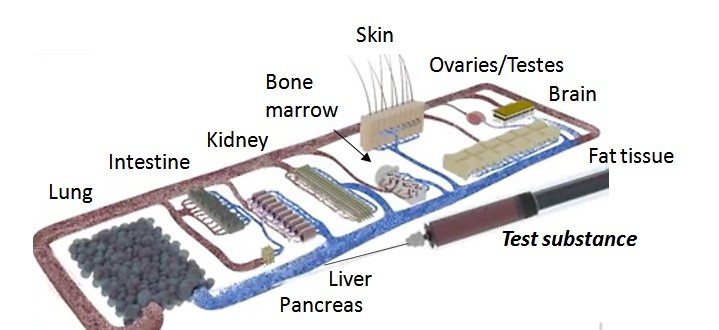
Ill. 4: 10 organs on a chip.
Illustration: TissUse.
The organ-like mini-structures not only possess the cell types that are found in the real organs and are necessary for their functions, but also the same “metabolic capacity” as the natural organ. The heart’s pump function is simulated by an integrated pump that generates a so-called pulsatile flow within the circulatory system. The use of cardiac muscle cells makes it possible to test potentially cardiotoxic substances.
In addition the possibility of administering substances orally (via the intestines), dermally (via the skin) or pulmonarily (via the lungs), a substance can also be directly introduced to the bloodstream. It is transported by the bloodstream to the liver, where it can be metabolised before being transported further. It passes from one organ to the next until it reaches its "target organ", during which process it is possible to study whether the target organ is affected or perhaps also another organ.
At present, the platform’s organoids can be repeatedly exposed to the test substance over a period of 28 days, as is the case in repeated dose-studies4. The corresponds to the subacute toxicity studies according to the OECD test guidelines5,6. Thus the active agent dose can be applied every day. Blood or tissue samples can be taken at regular intervals, just as in animal tests. At the end of the 28-day exposure period, the treated organoid cultures are removed and studied.
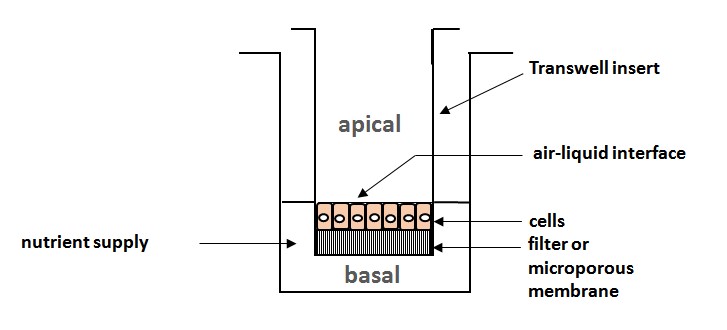
Ill. 5: Schematic of a transwell assay, the classic variant of cell cultivation for exposure: The cells are sited on a microporous membrane in transwell migration inlays; all life-supporting functions such as nutrient medium, temperature, gases, etc. are ensured; the surfaces of skin and lung tissue cells have immediate contact to the ambient atmosphere, as in natural conditions, whilst being provided with nutrients via the membrane on the basal side.
Schematic: Christiane Hohensee
How are mini-organs generated?
Cell lines or primary cells can be a suitable starting point, and direct biopsies can also be cultivated. One can also use precursor cells for a specific cell type or induced pluripotent stem cells harvested from differentiated tissues, then reprogrammed and differentiated to the desired cell type. These cell types are cultivated in various nutrient media with growth and/or differentiation factors. Shaping microtissues, for instance as spheroids with a diameter of 100-500 micrometres, has proven to be successful7. Spheroids can be developed in a hanging drop culture. Taking the liver as an example, these spheroids contains all cell types also found in a real liver, e.g. hepatocytes, endothelial and immune cells (Kupffer cells). It is not the shape that is decisive, but rather their functionality, which allows them to react to test substances like real organs.
Other systems have also proven to be effective, for instance a string-like arrangement of hepatocytes that maintains the natural polarity of the liver cells and allows a separation of the bile-producing cells7. However, the organoids currently only approximate the characteristics of tissue biopsies (left over from surgery on patients)7, but they can be cultivated for 28 days.
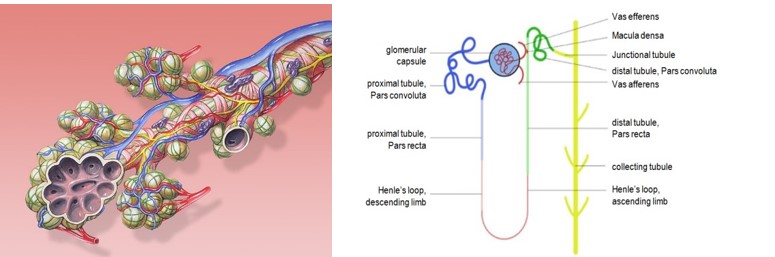
A mammalian organ is built up from a multitude of small units that have the same functions within the organ. This is what makes it possible to make build a miniature organism with comprehensive organ functions in the first place. These units are for instance the nephron in the kidneys, the alveoli in the lungs and the lobules in the liver (Ill. 6, 8 and 9).
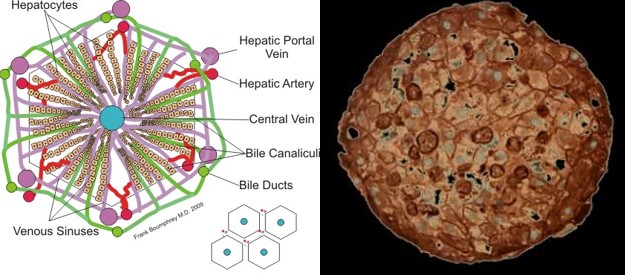
Ill. 6: The smallest unit in the liver: the lobule. Illustration: Boumphreyfr
Ill. 7: Cross section of a liver sphere. Photo: Insphero AG.
Ill. 8: The smalles unit in the lung: the alveolus. Illustration: Patrick J. Lynch.
Ill. 9: The smallest unit in the kidney: the nephron. Schematic: Calibu28.
The platforms can also be used for mounting organoids from mammalian cells, in order to draw comparisons between already published animal test results. The goal, however, is a human-specific system, as the test results are generally intended to benefit humans, too.
The final version of the Human-on-a-Chip to be developed is intended to be exposed to multiple substances and tested simultaneously in large numbers. This can deliver more reliable data for evaluating the safety and efficiency of substances compared to animal tests, before humans are exposed to them, thus considerably saving costs and time.

Ill. 10: The goal is to have a large number of small Human-on-a-Chip systems running simultaneously. This would save a considerable amount of time and thus also expenses.
Illustration: TissUse.
How many animals can be replaced?
The long-term goal is to be able to replace as far as possible all animal tests in the areas of acute, subchronic and chronic substance testing (see graph). Taking the numbers of animals used for “disease models”, the number would even be much higher (see interview below).
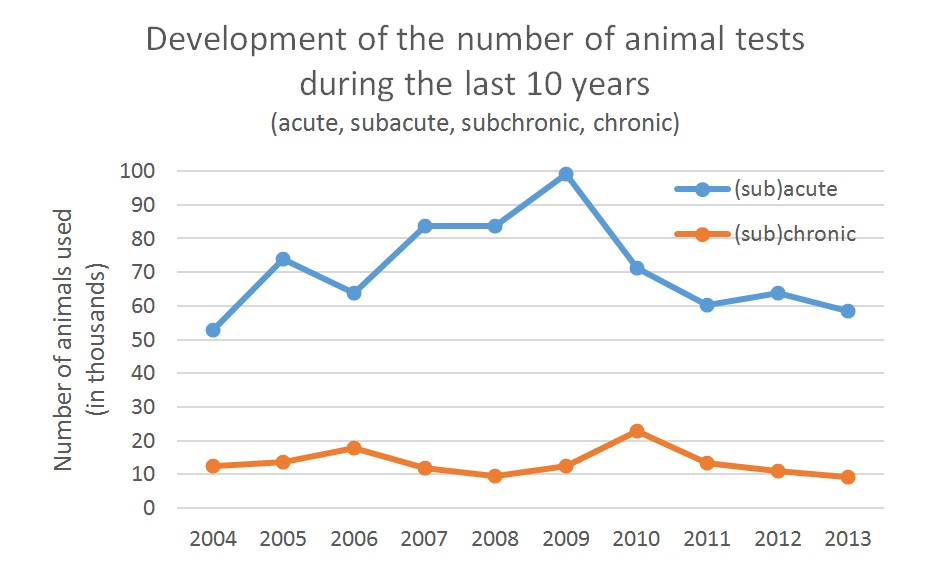
Ill. 11: Animal test statistics for 2013 from the German Federal Ministry of Food and Agriculture: The numbers for acute and chronic toxicity testing have only sunk slightly so far. Graph: Christiane Hohensee
According to statistics on animal testing in Germany, the reduction in the number of animals tested on is only slight8. Going by the numbers for 2013, it would potentially be possible to replace more than 67,000 animals in Germany alone using the Multi-Organ-on-a-Chip. On a global scale the numbers would of course be much higher (see interview below).
Table 1: Summary of Test Guidelines for Studying Systemic Toxicity
OECD Test Guidelines5,6
TG Nr. Description
402 Acute Toxicity (dermal)
403 Acute Inhalation Toxicity Study
425 Acute Toxicity (oral), Up-and-Down Procedure (UDP)
420 Acute Oral Toxicity – Fixed Dose Procedure
423 Acute Oral Toxicity – Acute Toxic Class Method
412 Subacute Inhalation Toxicity: Repeated Dose Inhalation Toxicity 14‐day or 28-Day Study
407 Subacute: Repeated Dose 28‐Day Oral Toxicity Study in Rodents
410 Subacute: Repeated Dose Dermal Toxicity: 21/28‐Day Study
408 Subchronic Study: Repeated Dose 90‐Day Oral Toxicity Study in Rodents
411 Subchronic Dermal Toxicity: 90‐Day Study
413 Subchronic Inhalation Toxicity: 90‐Day Study
452 Chronic Toxicity Study
How much time must be reckoned with?
Work is underway to start the first studies in 2018. This includes a possibility for validation15, with several international laboratories unaware of one another, using this technology to test substances they are not familiar and if possible arriving at comparable results.
If that is the case, these platforms can be used for large-scale high-throughput systemic toxicity tests of substances as a new replacement method for animal experiments.
Medium and long-term perspective: disease models and basic research
One use of the Human-on-a-Chip for emulating disease models is already planned for the first studies. Once the platform with healthy organoids is established, it will only be a small step to develop complex disease models of the liver, the kidneys, the brain, etc.
Pilot projects already exist9, 10,11.
… “an open innovation approach” …
InVitro+Jobs spoke with Dr. Reyk Horland about the current stage of development and the prospects for the Human-on-a-Chip.
InVitro+Jobs: How seriously was the idea of creating an artificial human test organism initially taken?
Reyk Horland: In 2010 we were more or less unique. The German evaluators demanded the project from the very beginning within the framework of the “GoBio” Initiative. In 2012 the Americans presented the idea of the “Human-on-a-Chip”. The organisations involved are DARPA12, NIH13 and the FDA14. What is unique is the participation of the FDA as advising authority, in order to introduce regulatory requirements for the technology at an early stage. A sum of about 140 million US dollars for 5 years and two competing groups (the MIT and the Wyss Institute) is of course a considerable investment compared to European funding. Different working groups are developing the individual organoids for the platforms.
InVitro+Jobs: Who else is working on a Human-on-a-Chip?
Reyk Horland: In the meantime there is research around the world, for instance with very powerful initiatives in Switzerland, the Netherlands and Russia.
InVitro+Jobs: Are there differences between the approaches of the international research groups?
Reyk Horland: There are two different trends to be observed here: 1.) The development of single organ solutions (Organ-on-a-Chip), which would later on be connected to create a common circulatory system or 2.) The development of a universal multi-organ platform technology that would allow the cultivation of several organ models in the correct tissue-to-tissue and tissue-to-fluid relationship. This is the approach we are working on.
InVitro+Jobs: The tasks are very comprehensive. Are you able to manage all these developments on your own? Who else is working on them with you?
Reyk Horland: Developing Human-on-a-Chip systems requires an extremely interdisciplinary approach. We have very close co-operation, for instance with engineers in the fields of microfluidics, measurement and control technology. The exciting thing about this “open innovation approach” is that you’re collaborating with very different experts whilst pursuing a common goal.
InVitro+Jobs: Which endpoints can be measured with your chip system?
Reyk Horland: The endpoints are much the same as in animal experiments. Just as when we take a blood sample, we can test medium for all physiologically relevant parameters, such as viability of the cells, stress, alterations in metabolic products. At the end, the organoids are removed, similar to organ removal in animals, and the cells are investigated for damage, inflammation, gene expression, RNA, etc.
InVitro+Jobs: How can small vessels be lined in such a miniature system? They need to have the same elasticity as in humans.
Reyk Horland: We construct miniature artificial blood vessels lined with human endothelial cells. This makes the vessels react to substances and human blood with its proteins flowing past the same way human blood vessels do.
InVitro+Jobs: Why is fat tissue one of the organs intended for use? It’s not actually an organ, is it?
Reyk Horland: Fat tissue has important storage and metabolic functions and is thus interesting for emulating disease models.
InVitro+Jobs: How many animals do you think your development could save?
Reyk Horland: EU data from 2011 counts 800,000 mammals just for chemical toxicity tests. Add to that another 5 million for the efficiency evaluation of pharmaceutical active agents. Not all animal models can be done away with: for instance, cognitive evaluations for neurodegenerative diseases can’t be replaced.
InVitro+Jobs: When do you think the complete Human-on-a-Chip will be ready for use?
Reyk Horland: The first prototype should be available for the first studies in 2018.
InVitro+Jobs: What does the funding situation currently look like?
Reyk Horland: So far, at the end of the first phase of “GoBio” funding, the system has worked excellently. We are currently at the point of investing in the spin-off16 for the second phase of funding. We are also attempting to acquire third-party finances, for instance via the European Horizon 2020 calls for proposals.
InVitro+Jobs: If students want to apply for a position with you, what are the prerequisites?
Reyk Horland: At the moment there is a great need for expertise in cell cultures, in order to establish organ models on the chip. Important prior knowledge would be experience cultivating human cells from primary cells and with their needs. That said, we are happy about every applicant who wants to use their expertise to make a contribution toward developing the technology.
Experience with microfluidics is also important, to be able to design new chip characteristics. Our cooperation partner, the Fraunhofer IWS, requires knowledge in microfluidics, electrical engineering and apparatus engineering.
InVitro+Jobs: Thank you for the interview.
Literature and websites:
1 www.tissuse.com
2 http://invitrojobs.kommzept.de/index.php/de/neuigkeiten/news-archiv/item/1558-berlin-33-tierschutzforschungspreis-des-bmel-verliehen
3 TissUse (2014): Multi-organ-chips revolutionize drug testing. Press release from 13 August 2014.
4 https://eurl-ecvam.jrc.ec.europa.eu/news/acute-mammalian-systemic-toxicity-testing-eurl-ecvam-releases-its-strategy
5 www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-4-health-effects_20745788
6 http://alttox.org/mapp/toxicity-endpoints-tests/acute-systemic-toxicity
7 Materne, E., Tonevitsky, A.G. & Marx, U. (2013): Chip-based liver equivalents for toxicity testing – organotypicalness versus cost-efficient high throughput. Lab on a Chip 13: 3481-3495.
8 Animal test statistics, German Federal Ministry of Food and Agriculture (2013) (German): www.bmel.de/SharedDocs/Downloads/Tier/Tierschutz/2013-TierversuchszahlenGesamt.pdf
9 www.sciencenews.org/article/mini-stomachs-grown-lab
10 http://invitrojobs.kommzept.de/index.php/en/news/news-archive/item/1207-lung-on-a-chip-simulates-human-lung-disease
11 http://invitrojobs.kommzept.de/index.php/en/news/news-archive/item/1030-boston-alzheimer-s-disease-model-of-human-cell-cultures
12 EPA: U.S. Environmental Protection Agency, http://www.epa.gov
13 NIH: U.S. National Institute of Health, http://www.nih.gov
14 FDA: U.S. Food and Drug Administration, http://www.fda.gov
15 Validation: A process of establishing documentary evidence that a process or system carried out in production or testing maintains the desired level of compliance at all stages. (cf. https://en.wikipedia.org/wiki/Validation_%28drug_manufacture%29)
16 Spin-off: A subsidiary, an outsourced organisational department of a company or a university.
Also see the working groups publications; these are listed on InVitro+Jobs:
http://invitrojobs.kommzept.de/index.php/en/technologies/lab-on-a-chip-systems
