Injury to a blood vessel leads to the release of thrombin from prothrombin via a signal cascade in the blood plasma. The enzyme activates clotting factors and fibrin closing the wound with their help. However, these processes located in blood vessels can lead to blood clots (thrombus) and other complications. Amongst others, thrombin activates the PAR-1 receptor, which leads to the activation and aggregation of platelets.
The antagonist to thrombin is protein C. It is normally activated after thrombin binds to thrombomodulin located on the endothelial cells of the inner wall of the blood vessels (1). It has an anti-inflammatory effect on the endothelial cells covering the blood vessel wall and dilutes the blood again. In a modified form, protein C is also administered as a drug, e. g. in the case of a heart attack or occlusion of a leg artery. However, this may, under certain circumstances, cause an excessively inhibition of the thrombin leading eventually to bleeding (2).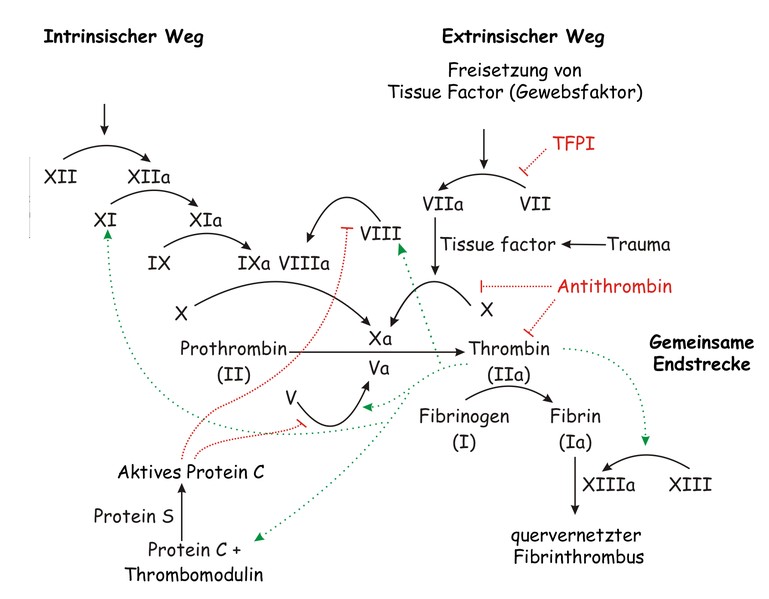
The process of secondary blood coagulation. Joe D, Wikipedia, 2007
In the group's work on hemostasis and thrombosis at the Beth Israel Deaconess Medical Center, in collaboration with the Wyss Institute at Harvard University in Boston, small molecules called parmodulins were tested which were able to simulate the activated protein C thus showing an anti-inflammatory and anti-thrombotic effect, without influencing the signalling pathway mediated by activated protein C as well as without affecting the endothelium (3).
They used an in vitro test, the blood vessel-on-a-chip. On this chip, smallest microchannels are milled in parallel, covered with collagen and lined with human endothelial cells. Thereby they were able to simulate the inner wall of human blood vessels. Whole blood and other substances can be passed through the small channels. The researchers first incubated the in vitro cultivated endothelial cells with their parmodulin for several hours, followed by an exposition of the cells to lipopolysaccharides or the messenger TNF-a to induce the induction of trombin. Compared to the controls, thrombin formation
was reduced by half. The small molecules had no effect on the clotting factor V and X of the coagulation cascade, which are responsible for blood coagulation. Therefore no bleeding phenomena occured.
The model was used to investigate the effect of platelet aggregation inhibitors (Parmodulins) on endothelial cells and not only to determine which signalling pathways are involved in parmodulin, but also to show that parmodulin protects endothelial cells from damage caused by inflammatory processes.
The research team has published its work in the Journal Proceedings of the National Academy of Sciences.
Source:
https://wyss.harvard.edu/stop-the-clots-spare-the-coagulation/
(1) http://flexikon.doccheck.com/de/Protein_C
(2) http://www.pharmawiki.ch/wiki/index.php?wiki=Vorapaxar
(3) Omozuanvbo Aisiku, Christian G. Peters, Karen De Ceunynck, Chandra C. Ghosh et al. (2015): Parmodulins inhibit thrombus formation without inducing endothelial injury caused by vorapaxar. Blood. 125 (12): 1976-1985.




 Dr. rer. nat.
Dr. rer. nat. Menschen für Tierrechte - Tierversuchsgegner Rheinland-Pfalz e.V.
Menschen für Tierrechte - Tierversuchsgegner Rheinland-Pfalz e.V.