“… It’s about better science …”
Impressions from the 10th World Congress in Seattle
The Congress was organised by the Institute for Risk Analysis and Risk Communication at the University of Washington in cooperation with the Environmental Protection Agency (EPA), the Center for Alternatives to Animal Testing (CAAT) at the Johns Hopkins Bloomberg School of Public Health, and the Alternative Congress Trust (ACT). ACT’s mission is to familiarise scientists and the public with alternatives to animal testing and make sure the new methods are widely disseminated. ACT’s CEO is Dr. Herman Koëter, generally known as the Chairman of the Netherlands National Committee for the protection of animals used for scientific purposes (NCad), which made a name for itself in the field of regulatory toxicology in December 2016 with its national plan for phasing out animal procedures in regulatory toxicology.

Seattle skyline.
Photo: Christiane Hohensee
About 700 abstracts from 43 countries were submitted, including some 350 posters. The contributions were spread across the nine different conference topics, such as innovative models for testing safety and efficacy, systems biology, ethics, translation, to name a few.
In the following are just a few examples of the developments presented at the congress.
Skin models now used worldwide
A major topic was in vitro models for investigating skin irritant substances and allergic reactions. Whereas such models are already part of OECD-approved graded test systems in Europe, their test results have just been reported for implementation in legislation in Brazil or China. A research team presented in vitro test results on skin irritation and skin corrosion from the EpiSkin Academy in China. As in Europe, the EpiSkin skin models are used there in a new integrated test strategy for assessing toxicological properties and cosmetics testing.
The models are thus on their way to being a global success story, with the cosmetics industry and companies such as L’Oréal and Beiersdorf playing a vital part in this development. L`Oréal contributed 23 conference papers on research collaboration.
There were also results from a skin sensitization validation study and a study on the transferability of the OECD-approved skin sensitisation tests to medical product testing.
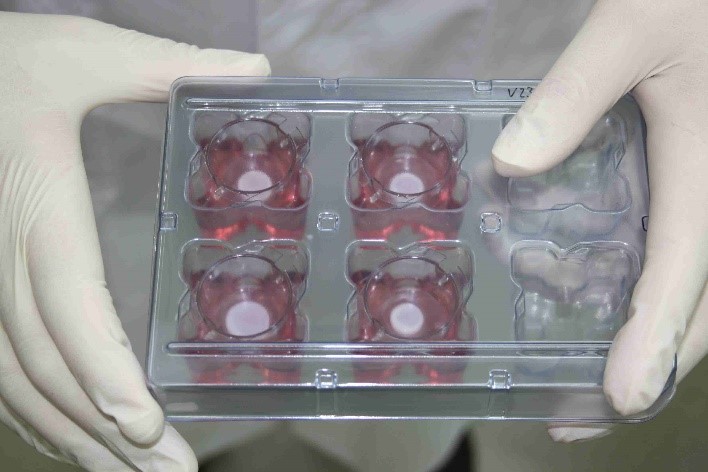
Cultured skin models in laboratory environment.
Photo: Christiane Hohensee
Business concerns involved in development and application of replacement methods
Many companies presented their own research into the development of replacement methods or provided some insight into their labs’ work. At the foremost was L’Òréal, which successfully tested a 4-organ chip in collaboration with the University of Central Florida.
The French company EpiSkin represented itself at the World Congress in Seattle. The company offers not only diverse constructed and whole skin models, but also cornea models, oral mucosa models and vaginal epithelial models for irritation tests. The cornea model was approved by the OECD in July after validation studies by both the ECVAM and Cosmetics Europe as the EpiOcular eye irritation test (EIT), as part of an integrated test strategy (no. 263, ENV/JM/MONO[2017]15). There is a new cell migration test with human corneal epithelial cells cocultivated with immune competent monocytes, whose migration through a membrane can be observed when inflammatory processes take place on the epithelial surface. Thus the possible in vitro tests now include the end point inflammatory processes.
BASF presented a method for detecting substances that can trigger allergic skin reactions. The new method can now also be used for predicting the potency of a substance’s skin-sensitising effect. This is important because chemicals must be classified and labelled in accordance with GHS.
The multi-technology company 3M also has a Strategic Toxicology Laboratory, in which products can be tested with numerous three-dimensional human tissues. These include skin and eye test models, as well as oral, pulmonary and vaginal tissues.
The company Qualyst Transporter Solutions has developed the so-called C-DILI Assay, with which the potential risk of a specific form of liver toxicity caused by obstructed bile flow (cholestatic hepatotoxicity) can be determined. Hepatocytes are cultivated in a sandwich culture; they form bile vesicles and produce all the necessary transporters. Hepatotoxicity is measured in terms of percentage increase of lactate dehydrogenase as a gauge for hepatic cell damage.
Developments in inhalation toxicology
Different research groups from Singapore, the USA and Austria presented test results with human lung cell cultures, cocultures with immune cells or a lung simulator. The latter exposes latex or porcine lungs to aerosols. All the research teams consider their different models suitable for testing lung-damaging chemicals.
The lung-on-a-chip model with human cell material von Prof. Donald Ingber from the Wyss Institute for Biologically Inspired Engineering at Harvard University has now reached the validation study stage.

Photo with aerosol: When can animal experiments be replaced in inhalation toxicity tests?
Photo: encierro, Fotolia.com
A German and Luxembourgish research team led by Prof. Brunhilde Blömeke from the Department of Environmental Toxicology at Trier University and Dr. Tommaso Serchi from the Luxembourg Institute of Science and Technology presented a very interesting model for replacing acute inhalation studies on animals. The scientists work with an immune competent human pulmonary cell model. A previously developed tetra cell culture with the alveolar cell line A549, differentiated dendrite-like cells (THP-1), mastocytes (HMC-1) and endothelial cells (EA.hy 926) on a porous membrane was augmented by a further immune cell type, macrophages. The immune cells form specific surface proteins (CD54 and TSLPr) and release certain messenger substances (cytokines), with which lung-irritating substances can reliably be distinguished from allergenic substances. So far there is no validated model for answering this question.
Dr. Samuel Constant, co-founder of the Swiss company Epithelix, is now able to replicate the entire human respiratory tract in vitro. Models of the nasopharynx, the trachea, bronchi and bronchioles (superficial and deeper lung layers) are combined with each other. The system can be cultivated for a period of six weeks.
Integrated test strategies for inhalation toxicity: identifying and developing the collective strength of test methods
As tests for acute inhalation toxicology require both local and systemic studies, the NICEATM organised a workshop with the PETA International Science Consortium (PISC) in September 2016, the results of which were presented at the Congress. The United States Environmental Protection Agency (EPA) and the Dow Chemical Company also took part in the workshop in September 2016. The participants recommended building a database with the available data on acute systemic toxicity; drafting a review of the most current toxicity mechanisms and test methods for acute inhalation toxicity; developing a computer-based decision tree; optimising the in vitro assays and developing standard protocols for their use in laboratories around the world (1). They described current test guidelines for inhalation toxicity as being merely basic principles that shed light on the fundamental test requirements. These must now be addressed using alternative approaches and the possibilities offered by information currently available, so as to be able to do away with the necessary tests. A multitude of alternative methods was seen as being already able to reliably identify cytotoxic substances, but a single method conducted on its own cannot be used to assess the diverse mechanisms of acute systemic inhalation toxicity. Therefore, integrated test and assessment methods are required, in order to cover the breadth of the different mechanisms and relevant chemicals, and to utilise the collective strengths of the most promising test methods and non-testing approaches. In order to be successful, input from branches of industry, academic disciplines, government agencies, stakeholder organisations and international organisations will be necessary.
Models for developmental and reproductive toxicity
Scientists at the EPA already use in vitro assays that detect toxicity mechanisms in cells and tissue, as well as computer prediction models, for investigating the effects of chemicals in the environment on progeny. Progress has been made with models capable of recapitulating angiogenesis, early renal development and palatogenesis. The next steps that are planned include the development stages of neurogenesis and neural tube closure, important events in embryonic development. All information should culminate in a virtual embryo.
A work group led by Prof. Elaine M. Faustman from the University of Washington School of Public Health in Seattle presented a three-dimensional in vitro coculture of murine testicle tissue for studying the effects of substances on a sensitive period of development. Over a longer period of time they measured the cell viability of Sertoli cells, Leydig cells und gametes, and testosterone production. The model allows the investigation of signal transduction pathways for cell division, steroid regulation and spermatogenesis in the development of the male reproductive organs. A research group at the University of Georgia led by Prof. Xiaozhong (John) Yu has developed a similar coculture model. They consider their model suitable for screening substances harmful to reproduction.
An Italian research group led by Prof. Paolo Antonio Netti from the Istituto Nazionale dei Tumori IRCCS in Milan has developed a human in vitro cervix model, with which the scientists intend to study cell interactions between epithelium and stroma tissue, and to test new therapeutics.
Other tissues on chips or microtiter plates
A Dutch group led by Dr. Stefan Vaessen from the University of Applied Sciences Utrecht presented a model of the human intestinal barrier. It comprises two compartments on a chip. The goal is to study drug absorption, metabolism in the intestinal wall, and the influences of mucosa and hormones.
The American company MatTek in Ashland, Massachusetts, has also worked on the development of an intestine model, using tissue from donors. They demonstrated that the tissue regenerates after injury. According to the developers, the cells can be cultured for up to 42 days.
Prof. Thomas Hartung from the Johns Hopkins University in Baltimore reported that immune cells can now be added to existing brain organoids, which comprise nerve and glia cells. This allows the scientists to study inflammatory processes in early stages of brain development. The group has also developed a cancer metastasis model for the brain’s nerve cells.
Multi-organ-on-a-chip: first results from tests and validation studies
Another important topic was multi-organ-on-a-chip technology, with results being presented from many collaborative studies between universities and the cosmetics industry. Companies are greatly interested in animal-free testing methods for long-term toxicity (repeated dose toxicity), after Europe banned animal experiments for the production and sales of cosmetics in 2013.
One example was a toxicokinetic study with a 2-organ chip comprising a skin model and 3D liver organoids. It tested changes in the concentration of tretinoin (all-trans retinoic acid or vitamin A acid) over time in the two organoid systems. Tretinoin is used in the cosmetics industry, but also in medicine, for instance for treating acne vulgaris (2). The substance was applied both once and repeatedly topically to the “skin” and systemically to the chip’s liver compartment. The researchers used the metabolism analyses to demonstrate the model’s functionality and intend to factor in the results along with other studies when extrapolating from in vitro to in vivo, because in vitro observations are not automatically applicable to the in vivo situation.
With the 4-organ chip developed by Prof. James Hickman from the University of Central Florida, systemic studies using cardiac and skeletal muscle tissue as well as nerve cells and liver tissue can be conducted over a period of 28 days, in which the influence of substances can be measured non-invasively by means of electrical and mechanical heart functions, neural activity, muscle contraction, liver enzymes, albumin and urea concentrations. The multi-organ-on-a-chip model works without a pump, as one problem with multi-organ-on-a-chip systems and simulating human organ physiology is designing the low-stress flow of “blood” or another suitable fluid; pumps exert such pressure on the miniature organs that this can lead to considerable stress in the cells, to damage and thus ultimately to false results. The pumpless system can be used to make physiological measurements without impairing cell viability. The working group has also developed a barrier-organ system, a renal cell system with proximal tubule cells and a blood-brain barrier system. All the cell culture systems are of human origin and free of any sera of animal origin.
Saskia Kliphus presents the new database of media without animal, developed by Animal-free Research UK and Universiteit Utrecht, Faculty of Veterinary Medicine.
Photo: Carolin Spicher.
Regulation and organ-on-a-chip technology: how much complexity is necessary?
The FDA still sees a number of technical challenges for a possible evaluation of organs on chips, as evaluation must be application-oriented. The short-term to middle-term field of application is therefore seen to be primarily in the area of screening.
The regulatory authorities still do not know how well the multi-organ chips work or how complicated a model must be, and in part they also do not know what requirements must be defined and whether simple systems would be sufficient. There is no data on how biomarkers should be measured and which biomarkers would be needed.
Prof. Hajime Kojima from the National Institute of Health Science in Tokyo recently started working with lab-on-a-chip technology and expects an evaluation from the FDA as to whether it will be possible in the future to use these models for regulatory purposes.
Prof. Bob van den Water pointed out as speaker of the European project EU-ToxRisk that precisely that is the project’s ultimate goal, to answer the question whether simpler cell models can also answer the necessary regulatory questions. Each model must show whether it can actually also represent human physiology.
Prof. Spielmann from the Freie Universität Berlin commented that the industry wishes to have such an evaluation.
Dr. Reyk Horland from TissUse emphasised the importance of quality standards. He said that TissUse’s assays are functional and can answer all concerned parties’ questions.
Extending tissue development to animal organs for the sake of comparison with the results of in vivo tests on animals, as suggested by Dr. Warren Casey, would not be necessary, as there is sufficient clinical human data available.
Researchers from the University of Washington spoke out in support of complex models, as these would deliver a wealth of valuable information.
However, standardising phenotypes and protocols would be essential, as there are so many protocols in laboratories around the world.
Conclusion:
At present the comparability of results from the laboratories and research facilities around the world is critical. There is still no standardisation for cells, culture conditions, media etc. Scientists at the Johns Hopkins University have also analysed batches of a commercially available human mammary adenocarcinoma cell line and found a remarkable phenotypical heterogeneity. The authors, Kleensang et al., concluded that this could have a considerable effect on the reproducibility of tests (3). The insights from validation studies over the past years also culminated in a new validation approach: Developments and the approval of methods do not progress linearly, but rather circularly, as new questions often arise during and after the validation process, making improved methods necessary (e.g. when the test method is also intended to be available for use with other substances that were not previously the subject of the validation).
Further information:
(1) https://ntp.niehs.nih.gov/pubhealth/evalatm/test-method-evaluations/acute-systemic-tox/acute-inhalation-toxicity/index.html
(2) http://flexikon.doccheck.com/de/Tretinoin
(3) Kleensang, A., Vantangoli, M. M., Odwin-DaCosta, S., Andersen, M. E., Boekelheide, K., Bouhifd, M., Fornace Jr, A. J., Li, H.-H., Livi, C. B., Madnick, S., Maertens, A., Rosenberg, M., Yager, J. D., Zhao, L. & Hartung, T. (2016): Genetic variability in a frozen batch of MCF-7 cells invisible in routine authentication affecting cell function. Scientific Reports 6, Article number: 28994. doi:10.1038/srep28994.
Congress abstract book:
http://www.altex.ch/altex-proceedings/wc-10-seattle




 Dr. rer. nat.
Dr. rer. nat. Menschen für Tierrechte - Tierversuchsgegner Rheinland-Pfalz e.V.
Menschen für Tierrechte - Tierversuchsgegner Rheinland-Pfalz e.V.